
Visby Medical
Founded Year
2012Stage
Grant - IV | AliveTotal Raised
$465.13MValuation
$0000Mosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
-106 points in the past 30 days
About Visby Medical
Visby Medical develops polymerase chain reaction (PCR) based diagnostic tests for the detection of infectious diseases. The company offers medical solutions for sexual health tests, respiratory health tests, and other infectious diseases. It was formerly known as Click Diagnostics. The company was founded in 2012 and is based in San Jose, California.
Loading...
Loading...
Expert Collections containing Visby Medical
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Visby Medical is included in 1 Expert Collection, including Unicorns- Billion Dollar Startups.
Unicorns- Billion Dollar Startups
1,244 items
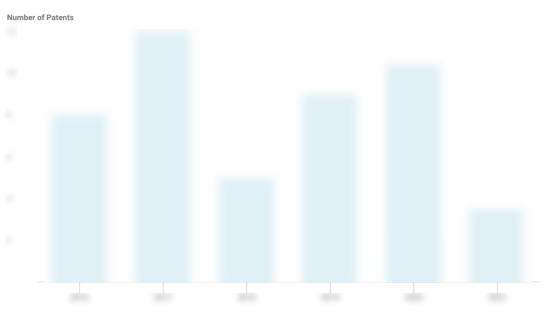
Visby Medical Patents
Visby Medical has filed 35 patents.
The 3 most popular patent topics include:
- medical tests
- molecular biology
- blood tests
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
10/22/2021 | 7/16/2024 | Molecular biology, Small nuclear RNA, Medical tests, RNA, Biotechnology | Grant |
Application Date | 10/22/2021 |
|---|---|
Grant Date | 7/16/2024 |
Title | |
Related Topics | Molecular biology, Small nuclear RNA, Medical tests, RNA, Biotechnology |
Status | Grant |
Latest Visby Medical News
May 30, 2024
News provided by Share this article Share toX Point-of-Care test significantly shortens time from ED arrival to test results, treatment and discharge – significant improvements are seen in the use of antibiotics for the treatment of chlamydia and gonococcal infections in women Nationwide increases in sexually transmitted diseases and antibiotic resistance create the need for a paradigm shift from traditional lab-based molecular testing SAN JOSE, Calif., May 30, 2024 /PRNewswire/ -- Visby Medical™ and the Johns Hopkins Medicine Department of Emergency Medicine announced findings from a study evaluating a new approach to management of the three most common non-viral sexually transmitted infections (STI) in women. The study found that use of the Visby Medical Sexual Health Test, a point-of-care (POC) polymerase chain reaction (PCR) test, shortened time from specimen collection to STI result to only 47 minutes per patient, compared to an average of 25 hours for the standard of care (SOC) lab-processed molecular send-out tests. The Visby Medical test also resulted in significantly higher rates of appropriate treatment and lower rates of over-treatment with antibiotics for Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections, compared to SOCi. Full data were presented on May 17 at the 2024 annual meeting of the Society for Academic Emergency Medicine (SAEM). The Visby Medical Sexual Health Test is the only "instrument-free" POC test available in the U.S. that provides PCR results in under 30 minutes. In March 2023, the Visby Medical test received 510(k) clearance and was granted a CLIA waiver from the U.S. Food and Drug Administration for its second-generation POC test. An STI surveillance report published in 2024 by the U.S. Centers for Disease Control (CDC) found more than 2.5 million cases in 2022ii. At the same time, the rate of inappropriate use of antibiotics to treat STIs has contributed to antimicrobial resistant strains of NG, prompting the World Health Organization (WHO) to release new guidance to improve diagnosis of STIs, including POC tests, with special emphasis on reducing antimicrobial resistanceiii. "The rise in STIs has created a crisis for the nation's hospital emergency departments because the conventional send out tests do not provide results fast enough to inform treatment decisions during the patient visit. Rather than lose a potentially infected patient, clinicians must decide whether to treat before they have definitive results, which isn't ideal for anyone and contributes to antibiotic resistance," explained Gary Schoolnik, MD, an infectious disease expert, Chief Medical Officer at Visby Medical, and Professor of Medicine at Stanford University. "The dramatic improvements seen with the Visby Sexual Health test in testing time, ED visit duration, and in the use of antibiotics point the way toward a new best practice for STI testing. Implementation of a new rapid point-of-care testing standard of care would greatly benefit our hospitals, urgent care centers and, most importantly, women who seek treatment for this condition." The study, Management of Sexually Transmitted Infections in the Emergency Department: Evaluation of a Point-of-care Test, compared two approaches to testing female patients presenting to the Johns Hopkins Emergency Department in Baltimore, MD with potential STIs during two separate four-month study periods in 2022 and 2023. The first approach, SOC central laboratory testing with batched nucleic acid amplification testing (NAAT) (n=517 patients), and the second approach, the POC PCR Visby Medical Sexual Health Test (n=304 patients), were compared for rates of STIs identified, median time-to-result intervals between the two phases, and rates of appropriate treatment (including over and under treatment) based on CDC recommended guidelines. For patients testing positive (4.8% for CT, 2.7% for NG, 8.0% for trichomoniasis [TV], and 1.9% with co-infections), proportions of appropriate treatment were significantly higher among the POC group for CT (92.7% vs 75.1% p<0.001) and NG (87.1% vs 74.3% p<0.001). Proportions of over-treatment were significantly lower among the POC group for CT-negative (7.0% vs 25.2% p<0.001) and NG-negative (13.0% vs 25.5% p<0.001) patients. No significant differences between the two testing groups were seen for TV. Median time intervals were significantly lower for the POC group, including time from specimen collection to STI results (47.0 minutes vs 25 hours p<0.001), time from ED arrival to STI results (5.7 hours vs 33.9 hours p<0.001), and time from ED arrival to discharge (9.1 hours vs 11.9 hours p<0.001)iv. The study was conducted by researchers at Johns Hopkins University with support from Visby Medical. About Visby Medical™ Visby Medical is transforming the order of diagnosis and treatment for infectious diseases so clinicians can test, talk with, and treat the patient in a single visit. The Company developed a proprietary technology platform that is the world's first instrument-free, single-use PCR platform that fits in the palm of your hand and rapidly tests for serious infections. The Visby Medical Sexual Health Test for women is the first step in a robust pipeline that is accelerating the delivery of fast and accurate, palm-sized PCR diagnostics to the point of care, and eventually for use at home. For more information, visit www.visbymedical.com . Follow Visby Medical on LinkedIn . Media Contact:
Visby Medical Frequently Asked Questions (FAQ)
When was Visby Medical founded?
Visby Medical was founded in 2012.
Where is Visby Medical's headquarters?
Visby Medical's headquarters is located at 3010 North First Street, San Jose.
What is Visby Medical's latest funding round?
Visby Medical's latest funding round is Grant - IV.
How much did Visby Medical raise?
Visby Medical raised a total of $465.13M.
Who are the investors of Visby Medical?
Investors of Visby Medical include CARB-X, John Doerr, Pitango Venture Capital, Artiman Ventures, Blue Water Life Science Advisors and 22 more.
Who are Visby Medical's competitors?
Competitors of Visby Medical include Sense Biodetection and 1 more.
Loading...
Compare Visby Medical to Competitors
Nostics is a company focused on the development of diagnostic technology in the healthcare industry. The company offers a platform that provides rapid, accessible, and affordable testing for bacteria and fungi, utilizing advanced nanotechnology, AI, and Raman spectroscopy in a portable device. Nostics primarily serves the healthcare sector, with solutions designed for primary care, diagnostic labs, and research and biopharma. It is based in Amsterdam, Netherlands.

bioMerieux specializes in in vitro diagnostics within the healthcare sector, focusing on the development and manufacturing of diagnostic tests. The company offers a broad range of products that enable rapid and accurate detection of infectious diseases, antimicrobial resistance, and food safety concerns, as well as solutions for pharmaceutical quality control. These products serve various sectors including healthcare, food industry, and pharmaceuticals. It was founded in 1963 and is based in Marcy L'Etoile, France.
Oxford Nanopore Technologies specializes in deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) sequencing technology within the biotechnology sector. The company offers products and solutions for DNA and RNA sequencing, including library preparation, sequencing devices, flow cells, and data analysis tools. Its products enable the analysis of genetic material for research in areas such as microbiology, human genetics, cancer, and environmental studies. It was founded in 2005 and is based in Oxford, United Kingdom.

Cue Health serves as a healthcare technology company specializing in diagnostic testing within the health sector. The company offers a portable molecular testing platform that provides lab-quality, actionable health insights quickly and conveniently. Cue Health primarily serves the healthcare industry, including clinics and individuals seeking at-home diagnostic solutions. It was founded in 2010 and is based in San Diego, California. In May 2024, Cue Health filed for bankruptcy.
Lucira Health (NASDAQ: LDHX) develops disposable Point-of-Care molecular tests for the diagnosis of infectious diseases. It develops a testing platform that produces laboratory molecular testing using a battery-powered test. The tool helps to detect COVID-19 and influenza Types A and B diseases. The company was founded in 2013 and is based in Emeryville, California.
Sense Biodetection operates as a molecular diagnostics company. It designs and develops proprietary molecular and device technologies. The company serves hospitals, urgent care, nursing homes, workplace and campus health, community health, and travel sectors. The company was founded in 2014 and is based in Abingdon, U.K. In February 2023, Sense Biodetection was acquired by Sherlock Biosciences. The terms of the transaction were not disclosed.
Loading...